COVID-19 EMERGENCY
Webmaster
COVID-19 PROTOCOL Duplicate 3
PROTOCOL FOR THE DETECTION OF SARS-COV-2 BY RNA PRECIPITATION AND RT-Q-PCR
SOP 2.0v, 29th June 2020
I. Sample collection and inactivation
- The sample will be collected using two swabs, a nasopharyngeal swab and a pharyngeal swab.
- Both swabs shall be introduced into the collection tube containing 2 mL of lysis buffer: Viral Lysis Buffer, NZYTech, Ref. MB40801 diluted 1:1 in PBS.
- After closing the tube it will be cleaned externally with a 10% bleach solution.
II. RNA Precipitation
(Approx. time 1h)
- Sterilize the BS2 hood for 20 min with UV light.
- Centrifuge at 12,000 g for 10 min at 4°C.
- Recover 600 µl of supernatant and transfer to a 1.5 ml tube containing 600 µl isopropanol with 4 µl glycoblue (Stock: 15 mg/ml; Use: 50 µg/ml; Invitrogen #AM9515) chilled (stored at -20°C).
- Mix by inversion and incubate 10 min on ice.
- Centrifuge at 12,000 g for 10 min at 4°C.
- Discard supernatant.
- Add 500 µl of 75% cold ethanol (kept at -20°C).
- Centrifuge at 12,000 g for 5 min at 4°C.
- Remove ethanol and leave pellet to air dry for 5-10 min.
- Resuspend pellet in 40 µl of H2O molecular biology quality (DNAse and RNAse free, not treated with DEPC), heat at 60ºC for 5 min.
- Transfer to a 96-well plate (plate nomenclature: "date-operator name initials-plate No": 090420-DM-P1) put an adhesive to cover it and store at -80C if it is not going to be used immediately. If the qPCR is to be run shortly, keep on ice.
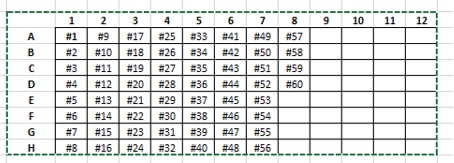
III. Implementation of RT-Q-PCR by fluorogenic-labeled probes on 384-well plates
(Approx. time 3h)
- NZYTech Speedy One-step RT-qPCR Probe Master Mix (2x, NZYTech #MB40503), TaqMan CDC 2019-nCoV RUO Kit Probes (N1 and RNAseP probes only, CDC #225397445) and a custom designed probe and primer set targeting RdRP gene (FW: 5’- GCCACAACTGCTTATGCTAATA-3’; RV: CGGACATACTTATCGGCAATTT; Probe: 5'FAM-TGTCAAGCT/ZEN/GTCACGGCCAATGTT-IABkFQ-3'; IDT) will be used.
- Final reaction volume will be 10 µl (8 µl TaqMan mix + 2 µl RNA sample) for 384-well plates. TaqMan mix will be performed as follows (volumes per well):
- 5µl NZY master mix (2x)
- 0.75 µl probe/1 µl probe for RdRP custom assay
- 2.25 µl H2O/ 2 µl H2O for RdRP custom assay (DNAse and RNAse free, not treated with DEPC)
Note: In case of detection using Life Technologies QS5 (Applied) thermal cycler or any other that requires ROX, 0.2 µl per well, that will be discounted from the water volume, should be added (this would apply to the calculations in point 4).
-
In addition to the samples to be diagnosed and tested in duplicate, the following controls should be incorporated:
- NTC: Non template control - TaqMan mix + 2 µl H2O (DNAse and RNAse free, not treated with DEPC)
- Negative Control: (HeLa RNA or RNA from any human cell culture, not infected)
- Positive control: TaqMan mix + 2 µl of 2019-CoV Plasmid Control from CDC (200,000 copies/µl; CDC #225397446).
Dilutions: 10^-2, 10^-4, 10^-5 (4,000 - 40 - 4 copies per well, respectively) to see the limit of sensitivity of the probes for each gene. The dilutions will be made with DNAse and RNAse free H2O, not treated with DEPC.
- Design and preparation of the plate
- Sterilize the BS2 hood, 20 min with UV light
- For a plate of 60 samples in duplicate + controls, three mixtures should be prepared, one for each probe (N1, RdRP and RNaseP) with the following volumes:
- 800 µl TaqMan Mix
- 120 µl probes / 160 ul probes for RdRP custom assay
-
360 µl H2O / 320 µl H2O for RdRP custom assay
- If pipetting with a multichannel pipette, the mixtures are passed into strips of 8 tubes (160 µl per tube).
- Add 8 µl of the mixture per well.
- Subsequently, 2 µl of the sample or control is pipetted in duplicate into the appropriate wells, the plate is covered with an adhesive cap (Q-PCR compatible optics) and centrifuged again.
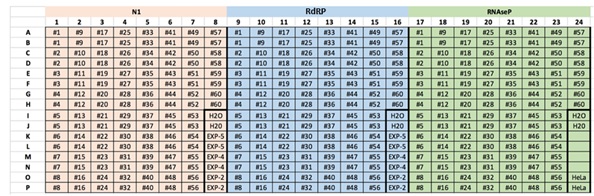
- The following amplification program will be used on a BioRad CFX384 touch thermal cycler, using the automatic threshold mode*:
| CYCLES | TEMPERATURE | TIME | REACTION |
|---|---|---|---|
| 1 | 50°C | 20 min | Retrotranscription |
| 1 | 95°C |
3 min |
Polymerase activation |
| 40 | 95°C | 5 sec | Denaturing |
| 55°C | 50 sec | Hybridisation + extension |
* Even if the automatic threshold and baseline mode is set, the curves will be checked before exporting the results in case there is too much background that could distort the results. It will be modified if necessary.
- Export results:
- Always send the data of the whole plate, even if there are empty wells
- Send the following files to the analysis group by email:
- The software's own (*.pcrd)
- Table with Ct values (Excel y csv)
IV. Analysis of the results (criteria)
This protocol summarizes the criteria for interpreting the results of the controls and clinical samples included in the COVID-19 diagnostic experiment. This criterion has been adopted during Phase 1 and has been modified according to the protocol published by the CDC.
Samples: 60 samples taken with the isopropanol precipitation protocol.
Probes: N1, RdRP, RNAseP
Controls:
- NTC: H2O (DNAse and RNAse free, not treated with DEPC)
- Negative Control: HeLa RNA (or RNA from any uninfected human cell culture)
- Positive Control: 2019-CoV Plasmid Control from CDC (200,000 copies/µl)
Dilutions: 10^-2 (around 1,000 CDC recommended), 10^-4, 10^-5 (dilutions near our detection limit (4,000 - 40 - 4 copies). Dilutions will be made with H2O free of DNAses and RNAses, not treated with DEPC.
Quality Control:
- NTC (H2O) must be negative for all 3 assays
- All clinical samples and negative controls should amplify RNAseP at Ct <35
- The positive control should amplify for the N1 and RdRP tests for all dilutions (10^-2, 10^-4, 10^-5).
INTERPRETATION OF RESULTS:
- The RNPase gene will be used as a control to validate the integrity of the sample
- In case RNPase comes up higher than 35 cycles (or undetermined) redo with SYBR.
- Samples that are positive for RNPase (fewer than 35 cycles) have more than 4 copies on N1, RdRP or both will be considered positive.
- Samples with more than 40 cycles for both N1 and RdRP (and RNPase with fewer than 35 cycles) will be considered negative.
- Samples with fewer than 4 copies for both N1 and RdRP (but fewer than 40 cycles) are considered doubtful. Likewise, samples in which the technical replicates are inconsistent (one replicate with more than 4 copies, and the other negative (Ct>40)) will be considered doubtful. In this case, and if after reviewing the amplification curves it is not possible to rule out a technical artefact, samples will be processed with the SYBR method described in section VI.
V. Validation of doubtful samples by RT-Q-PCR by SYBR
(Approx. time 3h)
Doubtful cases will be repeated with SYBR Green, and the primer set of the article by Won et al 2020.
- One-Step TB Green PrimeScript RT-PCR Kit II (Takara, #RR086-A) and primers from Won et al 2020 (RdRP, S, N and RPP30) will be used::
| SPECIES | TARGET | PRIMER NAME | PRIMER FORWARD (5’-3’) | PRIMER REVERSE (5’-3’) |
|---|---|---|---|---|
| SARS-CoV-2 | RdRP | SARS-CoV- 2_IBS_RdRP1 | CATGTGTGGCGGTTCACTAT | TGCATTAACATTGGCCGTGA |
| S | SARS-CoV- 2_IBS_S1 | CTACATGCACCAGCAACTGT | CACCTGTGCCTGTTAAACCA | |
| N | SARS-CoV-2_IBS_N1 | CAATGCTGCAATCGTGCTAC | GTTGCGACTACGTGATGAGG | |
| HUMAN | RPP30 | IBS_RPP30 | CTATTAATGTGGCGATTGACCGA | TGAGGGCACTGGAAATTGTAT |
- Final reaction volume will be 10 µl (8 µl Sybr Green mix + 2 µl RNA sample) for 384-well plates. TaqMan mix will be performed as follows (volumes per well):
- 5µl One Step TB Green RT-PCR Buffer 4 (2x)
- 0.4 µl PrimerScript 1 step Enzyme Mix 2
- 1 μl direct and reverse primer mix (5µM)
- 1.6 μl of H2O DNAse and RNAse free, not treated with DEPC
-
Reaction will be carried out as follows: 25°C (10 min), 50 °C (30 min), 85°C (5 min) and chill on ice.
- Add 8 μl of the mixture per well
- Subsequently, 2 μl of the sample or control will be pipetted in duplicate into the appropriate wells, the plate will be covered with an adhesive cap (optically compatible with the Q-PCR system) and centrifuged again.
- The following Life Technologies QS5 (Applied) amplification program will be used, using the automatic threshold mode:
| CYCLES | TEMPERATURE | TIME | REACTION |
|---|---|---|---|
| 1 | 42°C | 5 min | Retrotranscription |
| 1 | 95°C | 10 min | Polymerase activation |
| 40 | 95°C | 5 sec | Denaturing |
| 60°C | 20 sec | Hybridisation + extension | |
| 1 | 95°C | 0 sec; 20°C/sec | Melting analysis |
| 1 | 65°C | 15 sec; 20°C/sec | |
| 1 | 95°C | 0 sec; 0.1°C/sec |
- In addition to the samples to be diagnosed, which will be tested in duplicate, the following controls will be incorporated:
- NTC: Non template control - SYBR mix + 2 μl H2O (free of DNAses and RNAses, not treated with DEPC)
- Negative Control: (HeLa RNA or RNA from any human cell culture, not infected)
- Positive Control: 2019-CoV Plasmid Control from CDC (200,000 copies/μl).
Dilutions: 10^-2, 10^-3, 10^-4, 10^-5 (4,000 - 400 - 40 - 4 copies) to see the limit of sensitivity of the primers for each gene. The dilutions will be made with H2O free of DNAses and RNAses, not treated with DEPC.
- Export results:
- Always send the data of the whole plate, even if there are empty wells
- Send the following files to the analysis group by email:
- The software's own (*.pcrd)
- Table with Ct values (Excel and csv)
Interpretation of results:
- The viral genes N, S and RdpP will be analysed.
- The human gene RPP30 will be used as a control to validate the integrity of the sample.
- Samples in which at least two of the three viral genes are amplified and the RPP30 gene is amplified with fewer than 35 cycles will be considered positive.
- Samples are considered negative if they do not amplify any of the three genes (Ct>40) and the RPP30 gene amplifies with fewer than 35 cycles.
- Those that do not pass the criteria to be defined as negative or positive will be considered indeterminate. It will be recommended to take a second sample after a few days.
- If the RPP30 amplifies with more than 35 cycles (or does not amplify; Ct>40) the sample will be REPEATED, as it is considered of insufficient quality for the analysis.
We thank Prof Inés Pineda-Torra @UCL for translating this SOP to English #auzolana
COVID-19 PROTOCOL
PROTOCOL FOR THE DETECTION OF SARS-COV-2 BY RNA PRECIPITATION AND RT-Q-PCR
SOP 2.0v, 29th June 2020
I. Sample collection and inactivation
- The sample will be collected using two swabs, a nasopharyngeal swab and a pharyngeal swab.
- Both swabs shall be introduced into the collection tube containing 2 mL of lysis buffer: Viral Lysis Buffer, NZYTech, Ref. MB40801 diluted 1:1 in PBS.
- After closing the tube it will be cleaned externally with a 10% bleach solution.
II. RNA Precipitation
(Approx. time 1h)
- Sterilize the BS2 hood for 20 min with UV light.
- Centrifuge at 12,000 g for 10 min at 4°C.
- Recover 600 µl of supernatant and transfer to a 1.5 ml tube containing 600 µl isopropanol with 4 µl glycoblue (Stock: 15 mg/ml; Use: 50 µg/ml; Invitrogen #AM9515) chilled (stored at -20°C).
- Mix by inversion and incubate 10 min on ice.
- Centrifuge at 12,000 g for 10 min at 4°C.
- Discard supernatant.
- Add 500 µl of 75% cold ethanol (kept at -20°C).
- Centrifuge at 12,000 g for 5 min at 4°C.
- Remove ethanol and leave pellet to air dry for 5-10 min.
- Resuspend pellet in 40 µl of H2O molecular biology quality (DNAse and RNAse free, not treated with DEPC), heat at 60ºC for 5 min.
- Transfer to a 96-well plate (plate nomenclature: "date-operator name initials-plate No": 090420-DM-P1) put an adhesive to cover it and store at -80C if it is not going to be used immediately. If the qPCR is to be run shortly, keep on ice.

III. Implementation of RT-Q-PCR by fluorogenic-labeled probes on 384-well plates
(Approx. time 3h)
- NZYTech Speedy One-step RT-qPCR Probe Master Mix (2x, NZYTech #MB40503), TaqMan CDC 2019-nCoV RUO Kit Probes (N1 and RNAseP probes only, CDC #225397445) and a custom designed probe and primer set targeting RdRP gene (FW: 5’- GCCACAACTGCTTATGCTAATA-3’; RV: CGGACATACTTATCGGCAATTT; Probe: 5'FAM-TGTCAAGCT/ZEN/GTCACGGCCAATGTT-IABkFQ-3'; IDT) will be used.
- Final reaction volume will be 10 µl (8 µl TaqMan mix + 2 µl RNA sample) for 384-well plates. TaqMan mix will be performed as follows (volumes per well):
- 5µl NZY master mix (2x)
- 0.75 µl probe/1 µl probe for RdRP custom assay
- 2.25 µl H2O/ 2 µl H2O for RdRP custom assay (DNAse and RNAse free, not treated with DEPC)
Note: In case of detection using Life Technologies QS5 (Applied) thermal cycler or any other that requires ROX, 0.2 µl per well, that will be discounted from the water volume, should be added (this would apply to the calculations in point 4).
-
In addition to the samples to be diagnosed and tested in duplicate, the following controls should be incorporated:
- NTC: Non template control - TaqMan mix + 2 µl H2O (DNAse and RNAse free, not treated with DEPC)
- Negative Control: (HeLa RNA or RNA from any human cell culture, not infected)
- Positive control: TaqMan mix + 2 µl of 2019-CoV Plasmid Control from CDC (200,000 copies/µl; CDC #225397446).
Dilutions: 10^-2, 10^-4, 10^-5 (4,000 - 40 - 4 copies per well, respectively) to see the limit of sensitivity of the probes for each gene. The dilutions will be made with DNAse and RNAse free H2O, not treated with DEPC.
- Design and preparation of the plate
- Sterilize the BS2 hood, 20 min with UV light
- For a plate of 60 samples in duplicate + controls, three mixtures should be prepared, one for each probe (N1, RdRP and RNaseP) with the following volumes:
- 800 µl TaqMan Mix
- 120 µl probes / 160 ul probes for RdRP custom assay
-
360 µl H2O / 320 µl H2O for RdRP custom assay
- If pipetting with a multichannel pipette, the mixtures are passed into strips of 8 tubes (160 µl per tube).
- Add 8 µl of the mixture per well.
- Subsequently, 2 µl of the sample or control is pipetted in duplicate into the appropriate wells, the plate is covered with an adhesive cap (Q-PCR compatible optics) and centrifuged again.

- The following amplification program will be used on a BioRad CFX384 touch thermal cycler, using the automatic threshold mode*:
| CYCLES | TEMPERATURE | TIME | REACTION |
|---|---|---|---|
| 1 | 50°C | 20 min | Retrotranscription |
| 1 | 95°C |
3 min |
Polymerase activation |
| 40 | 95°C | 5 sec | Denaturing |
| 55°C | 50 sec | Hybridisation + extension |
* Even if the automatic threshold and baseline mode is set, the curves will be checked before exporting the results in case there is too much background that could distort the results. It will be modified if necessary.
- Export results:
- Always send the data of the whole plate, even if there are empty wells
- Send the following files to the analysis group by email:
- The software's own (*.pcrd)
- Table with Ct values (Excel y csv)
IV. Analysis of the results (criteria)
This protocol summarizes the criteria for interpreting the results of the controls and clinical samples included in the COVID-19 diagnostic experiment. This criterion has been adopted during Phase 1 and has been modified according to the protocol published by the CDC.
Samples: 60 samples taken with the isopropanol precipitation protocol.
Probes: N1, RdRP, RNAseP
Controls:
- NTC: H2O (DNAse and RNAse free, not treated with DEPC)
- Negative Control: HeLa RNA (or RNA from any uninfected human cell culture)
- Positive Control: 2019-CoV Plasmid Control from CDC (200,000 copies/µl)
Dilutions: 10^-2 (around 1,000 CDC recommended), 10^-4, 10^-5 (dilutions near our detection limit (4,000 - 40 - 4 copies). Dilutions will be made with H2O free of DNAses and RNAses, not treated with DEPC.
Quality Control:
- NTC (H2O) must be negative for all 3 assays
- All clinical samples and negative controls should amplify RNAseP at Ct <35
- The positive control should amplify for the N1 and RdRP tests for all dilutions (10^-2, 10^-4, 10^-5).
INTERPRETATION OF RESULTS:
- The RNPase gene will be used as a control to validate the integrity of the sample
- In case RNPase comes up higher than 35 cycles (or undetermined) redo with SYBR.
- Samples that are positive for RNPase (fewer than 35 cycles) have more than 4 copies on N1, RdRP or both will be considered positive.
- Samples with more than 40 cycles for both N1 and RdRP (and RNPase with fewer than 35 cycles) will be considered negative.
- Samples with fewer than 4 copies for both N1 and RdRP (but fewer than 40 cycles) are considered doubtful. Likewise, samples in which the technical replicates are inconsistent (one replicate with more than 4 copies, and the other negative (Ct>40)) will be considered doubtful. In this case, and if after reviewing the amplification curves it is not possible to rule out a technical artefact, samples will be processed with the SYBR method described in section VI.
V. Validation of doubtful samples by RT-Q-PCR by SYBR
(Approx. time 3h)
Doubtful cases will be repeated with SYBR Green, and the primer set of the article by Won et al 2020.
- One-Step TB Green PrimeScript RT-PCR Kit II (Takara, #RR086-A) and primers from Won et al 2020 (RdRP, S, N and RPP30) will be used::
| SPECIES | TARGET | PRIMER NAME | PRIMER FORWARD (5’-3’) | PRIMER REVERSE (5’-3’) |
|---|---|---|---|---|
| SARS-CoV-2 | RdRP | SARS-CoV- 2_IBS_RdRP1 | CATGTGTGGCGGTTCACTAT | TGCATTAACATTGGCCGTGA |
| S | SARS-CoV- 2_IBS_S1 | CTACATGCACCAGCAACTGT | CACCTGTGCCTGTTAAACCA | |
| N | SARS-CoV-2_IBS_N1 | CAATGCTGCAATCGTGCTAC | GTTGCGACTACGTGATGAGG | |
| HUMAN | RPP30 | IBS_RPP30 | CTATTAATGTGGCGATTGACCGA | TGAGGGCACTGGAAATTGTAT |
- Final reaction volume will be 10 µl (8 µl Sybr Green mix + 2 µl RNA sample) for 384-well plates. TaqMan mix will be performed as follows (volumes per well):
- 5µl One Step TB Green RT-PCR Buffer 4 (2x)
- 0.4 µl PrimerScript 1 step Enzyme Mix 2
- 1 μl direct and reverse primer mix (5µM)
- 1.6 μl of H2O DNAse and RNAse free, not treated with DEPC
-
Reaction will be carried out as follows: 25°C (10 min), 50 °C (30 min), 85°C (5 min) and chill on ice.
- Add 8 μl of the mixture per well
- Subsequently, 2 μl of the sample or control will be pipetted in duplicate into the appropriate wells, the plate will be covered with an adhesive cap (optically compatible with the Q-PCR system) and centrifuged again.
- The following Life Technologies QS5 (Applied) amplification program will be used, using the automatic threshold mode:
| CYCLES | TEMPERATURE | TIME | REACTION |
|---|---|---|---|
| 1 | 42°C | 5 min | Retrotranscription |
| 1 | 95°C | 10 min | Polymerase activation |
| 40 | 95°C | 5 sec | Denaturing |
| 60°C | 20 sec | Hybridisation + extension | |
| 1 | 95°C | 0 sec; 20°C/sec | Melting analysis |
| 1 | 65°C | 15 sec; 20°C/sec | |
| 1 | 95°C | 0 sec; 0.1°C/sec |
- In addition to the samples to be diagnosed, which will be tested in duplicate, the following controls will be incorporated:
- NTC: Non template control - SYBR mix + 2 μl H2O (free of DNAses and RNAses, not treated with DEPC)
- Negative Control: (HeLa RNA or RNA from any human cell culture, not infected)
- Positive Control: 2019-CoV Plasmid Control from CDC (200,000 copies/μl).
Dilutions: 10^-2, 10^-3, 10^-4, 10^-5 (4,000 - 400 - 40 - 4 copies) to see the limit of sensitivity of the primers for each gene. The dilutions will be made with H2O free of DNAses and RNAses, not treated with DEPC.
- Export results:
- Always send the data of the whole plate, even if there are empty wells
- Send the following files to the analysis group by email:
- The software's own (*.pcrd)
- Table with Ct values (Excel and csv)
Interpretation of results:
- The viral genes N, S and RdpP will be analysed.
- The human gene RPP30 will be used as a control to validate the integrity of the sample.
- Samples in which at least two of the three viral genes are amplified and the RPP30 gene is amplified with fewer than 35 cycles will be considered positive.
- Samples are considered negative if they do not amplify any of the three genes (Ct>40) and the RPP30 gene amplifies with fewer than 35 cycles.
- Those that do not pass the criteria to be defined as negative or positive will be considered indeterminate. It will be recommended to take a second sample after a few days.
- If the RPP30 amplifies with more than 35 cycles (or does not amplify; Ct>40) the sample will be REPEATED, as it is considered of insufficient quality for the analysis.
We thank Prof Inés Pineda-Torra @UCL for translating this SOP to English #auzolana
