Parkinson’s disease is currently the second most widespread neurogenerative pathology. Current therapies are mainly of a replacement type and pose problems in the long term, so the challenge is to establish an early diagnosis and develop neuroprotective and neurorestorative therapies that will allow the symptoms of the disease to be slowed down or even reversed. As part of the PhD thesis by Catalina Requejo, work conducted by the LaNCE Group of the Department of Neurosciences in the Faculty of Medicine and Nursing, and in which the Neuropharmacology group of the Faculty of Medicine and Nursing and the NanoBiocel group of the Faculty of Pharmacy were involved, documented the regenerative, neuroprotective effect of two neurotrophic factors when they are applied in a combined way.
The combination of two proteins exerts a regenerating, neuroprotective effect in Parkinson’s disease
According to a piece of research by the UPV/EHU-University of the Basque Country, the synergy between two neurotrophic factors would be beneficial particularly during an early phase of the disease
- Research
First publication date: 02/01/2018

Parkinson’s is a motor disorder caused by the loss of dopaminergic neurons in the black substance of the brain. These neurons are the nerve cells that produce dopamine, a neurotransmitter that plays a key role in the modulation of involuntary movements.
The research carried out at the UPV/EHU was developed in an experimental model that allows different stages of Parkinson’s disease to be reproduced. The results showed that the changes caused by the condition were not homogeneous in the different parts of the brain affected. “The impairment is correlated with the specific anatomic distribution of the dopaminergic neurons and their terminals,” pointed out the researcher Catalina Requejo. In other words, those areas of the black substance in which the dopaminergic neurons have more connections with regions that remain whole were found to be less affected.
After confirming that the experimental model could be used to explore the morphological and functional changes caused by the disease, therapeutic strategies based on the release of neurotrophic factors were applied. These factors are proteins that encourage cell growth, plasticity and survival, and therefore play an essential role in controlling neuronal function.
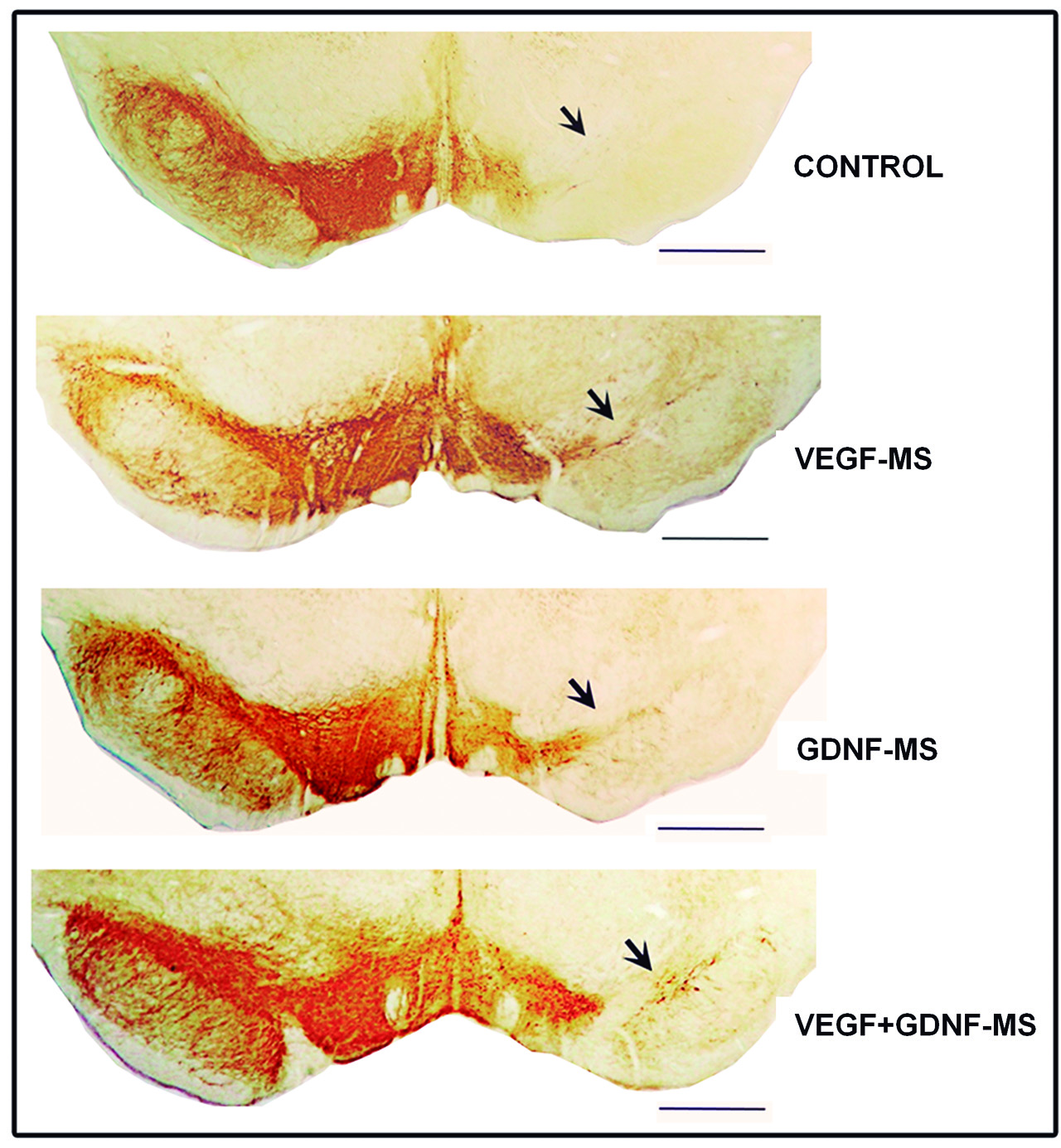
Specifically, two factors were applied: the Vascular Endothelial Growth Factor (VEGF) and the Glial Cell-derived Neurotrophic Factor (GDNF). These molecules were delivered encapsulated in microspheres or in nanospheres, even smaller than the former, comprising a biocompatible, biodegradable polymer: Poly Lactic-co-glycolic Acid (PLGA), which allows them to be released continuously and gradually. Furthermore, the factors were administered in a combined way to determine whether, together, they induced a synergistic effect.
The results were encouraging in both the early and severe phase of the model. The combining of the VEGF and GDNF not only significantly reduced the degeneration of the dopaminergic neurons of the black substance, it also induced the formation of new cells and cellular differentiation. The researchers were also able to confirm that there had been an improvement in the areas where the nerve fibres in this region were projected. To confirm the synergistic, neurogenerative effect of the two factors, they administered a molecule that inhibits the receptors of the two neurotrophic factors they were studying. “The consequences for the dopaminergic system were even worse, which supports the beneficial synergistic effects exerted by the VEFG and the GDNF in Parkinson’s,” concluded the researcher.
Finally, it is worth highlighting that the best results were obtained when the factors were delivered encapsulated in nanospheres during the early phase of the disease replicated in the model. All this reinforces the importance of early diagnosis and that “nanotechnology could be a very useful tool when it comes to administering neurotrophic factors,” she added.
Additional information
These pieces of work published in the prestigious scientific journal Molecular Neurobiology are the result of the PhD thesis by Catalina Requejo. Having obtained a degree in Biology from the UPV/EHU, she wrote up her PhD with international mention in Neurosciences achieving a Cum Laude distinction. Her PhD thesis, supervised by José Vicente Lafuente, tackled the morpho-functional analysis of the changes presented by an experimental model of Parkinson’s and the effects of administering neurotrophic factors in the said model. After a three-month, pre-doctoral placement at the Harvard Medical School in Boston, she received a two-year postdoctoral scholarship at the Mount Sinai Hospital in New York where she conducted a study into the molecular and cellular mechanisms involved in autophagy activation for synuclein degradation.
Bibliographic reference
- Nanoformulation: A Useful Therapeutic Strategy for Improving Neuroprotection and the Neurorestorative Potential in Experimental Models of Parkinson's Disease.
- Int Rev Neurobiol; 137:99-122.
- DOI: 10.1016/bs.irn.2017.09.003